InnAccel Technologies
Saans: Respiratory Support Device with CPAP Therapy for Infants and Neonates

IMPACT
500+
SAANS units deployed
2,500+
infants treated

Geographical Focus
- Assam, Chhattisgarh, Karnataka, Uttar Pradesh, Maharashtra, Rajasthan, and Andhra Pradesh

Nitesh Jangir Co-founder and Director, InnAccel Technologies

Our vision is to enable access to quality healthcare for everyone, regardless of the clinical setting. This vision is now closer to reality with the help of SAMRIDH’s support. The financial assistance and advisory support is playing a substantial role in our growth. We have deployed the Saans device to all public hospitals in Assam. This project has the potential to become a role model for the global implementation of non-invasive ventilation in limited- resource settings. The support from SAMRIDH has put us on an accelerated path to becoming financially sustainable.

Samridh support
SAMRIDH’s financial support has significantly boosted InnAccel’s manufacturing capacity for Saans devices, ensuring wider availability. Additionally, the support enabled the enterprise to establish a dedicated commercial and support team, driving the uptake of the device within both private and public healthcare settings. It also facilitated comprehensive training for healthcare professionals, ensuring effective use of the device. InnAccel has leveraged SAMRIDH’s support to raise further funding in the form of grants, and plan to raise equity, leading to a leverage of about 7x over SAMRIDH’s support. SAMRIDH’s blended financing approach promises to expand Saans’ reach, enhancing neonatal care and saving lives across India.
babies die annually in India during transport to NICUs due to inadequate respiratory support[1]
premature babies in India succumb annually to Respiratory Distress Syndrome (RDS)[2]
Respiratory Distress Syndrome (RDS) is a common breathing disorder among premature babies in India. While this condition can be treated with Continuous Positive Air Pressure (CPAP), a significant challenge exists because the majority of infants in India are born in primary or secondary care centers that lack Neonatal Intensive Care Units (NICUs) equipped to manage such emergencies. The existing CPAP solutions require NICU-grade infrastructure, rendering them unusable during transport. As a result, about a third of RDS-related infant deaths occur during transport to an NICU. To reduce these tragic losses, it is crucial to make CPAC therapy accessible in lower-level healthcare centers and suitable for transport to NICUs.
[1] Guardian, The Sunday. 2019. “ Saans: A portable device for neonatal breathing support – The Sunday Guardian Live.” April 6, 2019. The Sunday Guardian Live.
[2] ET HealthWorld 2019. “ InnAccel launches SAANS: portable neonatal CPAP device for infants with Respiratory Distress Syndrome.” April 4, 2019. ETHealthworld.com
Saans Device
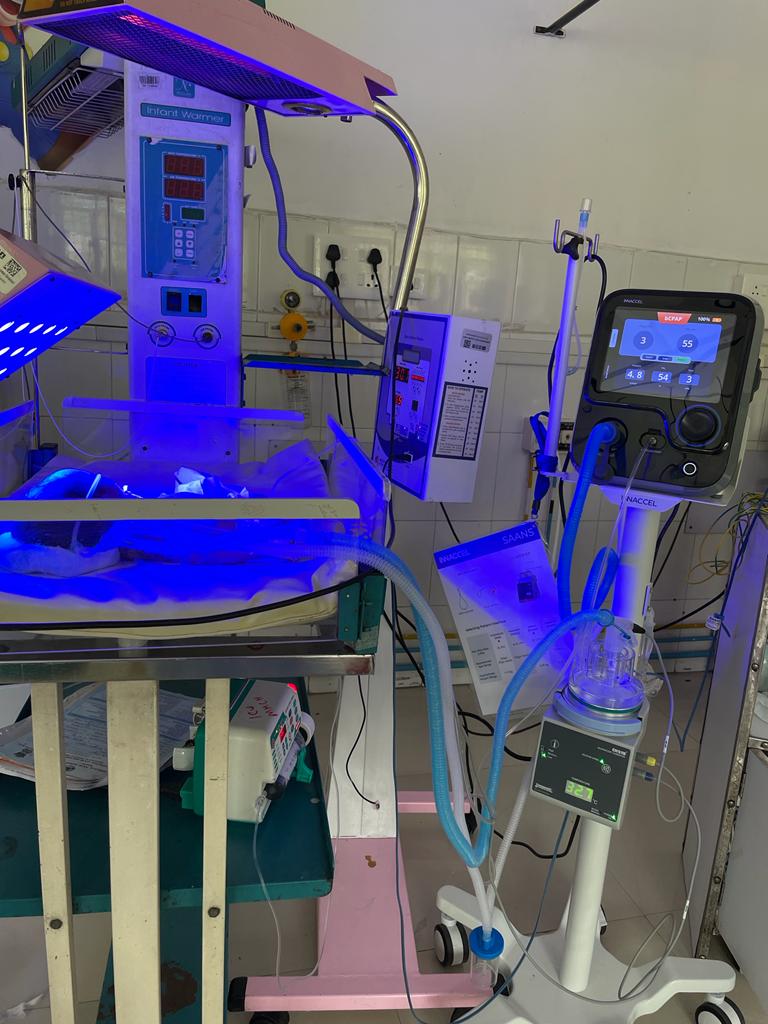
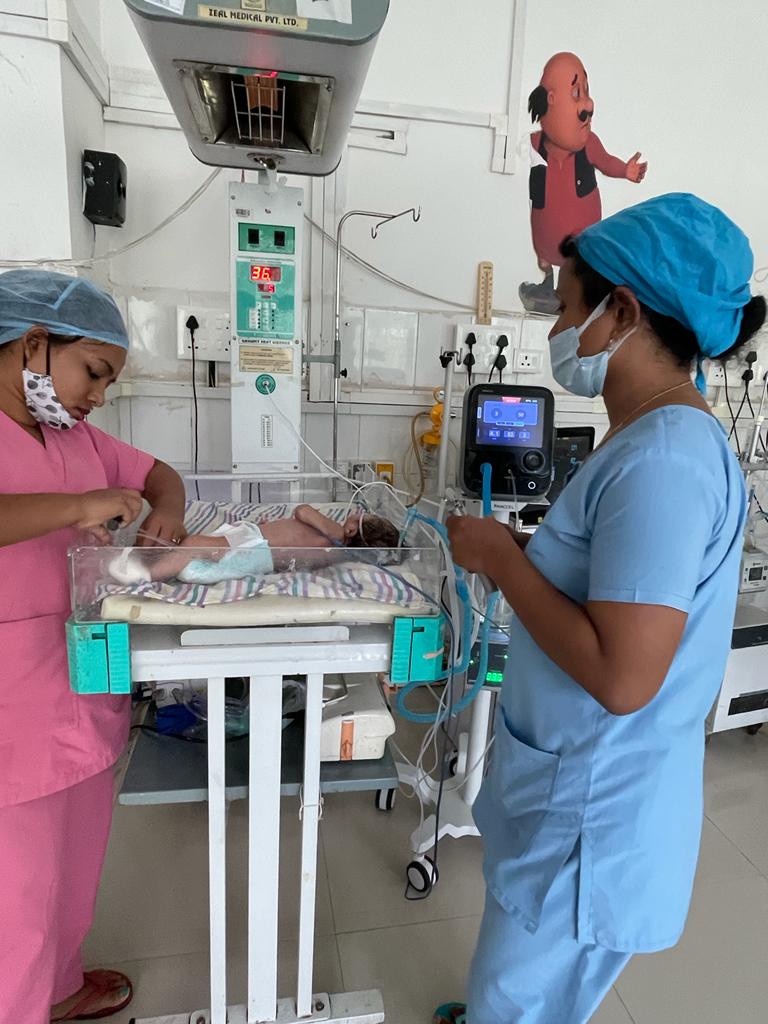
InnAccel Technologies’ Saans is a groundbreaking, infrastructureindependent, low-skill, and non-invasive neonatal Continuous Positive Airway Pressure (CPAP) device. Designed to offer shortterm breathing support for infants with Respiratory Distress Syndrome (RDS), Saans is a gamechanger in non-ICU settings. Its multi-powered, multi-mode design makes it exceptionally versatile, with a manual powering option and compatibility with standard oxygen sources. This device was conceptualized and developed in partnership with the Consortium of Affordable Medical Technologies (CAMTech) and the Department of Biotechnology, Government of India. Saans represents a significant advancement in ensuring accessible and effective breathing support for children, both during transport and in secondary care settings.

Resources
Key Stakeholders


